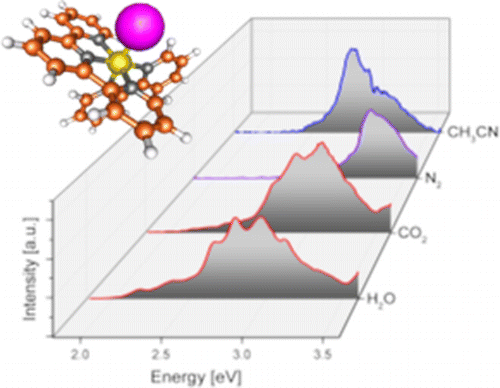
We report electronic spectra of a series of ruthenium polypyridine complexes of the form [(trpy)(bipy)RuII–L]2+ (bipy = 2,2′-bipyridine and trpy = 2,2′:6′,2″-terpyridine), where L represents a small molecular ligand that occupies the last coordination site. Species with L = H2O, CO2, CH3CN, and N2 were investigated in vacuo using photodissociation spectroscopy. All species exhibit bright metal-to-ligand charger transfer (MLCT) bands in the visible and near UV, but with different spectral envelopes and peak energies, encoding the influence of the ligand L on the electronic structure of the complex. Several individual electronic bands can be resolved for L = H2O and CO2, while the spectra for L = N2 and CH3CN are more congested, even at low ion temperatures. The experimental results are discussed in the framework of time-dependent density functional theory.
Shuang Xu†, James E. T. Smith‡, and J. Mathias Weber‡
† JILA and Department of Physics, University of Colorado, Boulder, Colorado 80309-0440, United States
‡ JILA and Department of Chemistry and Biochemistry, University of Colorado, Boulder, Colorado 80309-0440, United States